Like zinc molybdate (ZnMoO?), zinc tungstate is a compound composed of two transition metal elements and oxygen, gaining widespread attention from semiconductor researchers due to its unique structure and physicochemical properties. Note: Tungsten, zinc, and molybdenum are all transition metal elements; tungsten and molybdenum belong to the same subgroup, with atomic numbers 74 and 42, respectively, while zinc has an atomic number of 30.
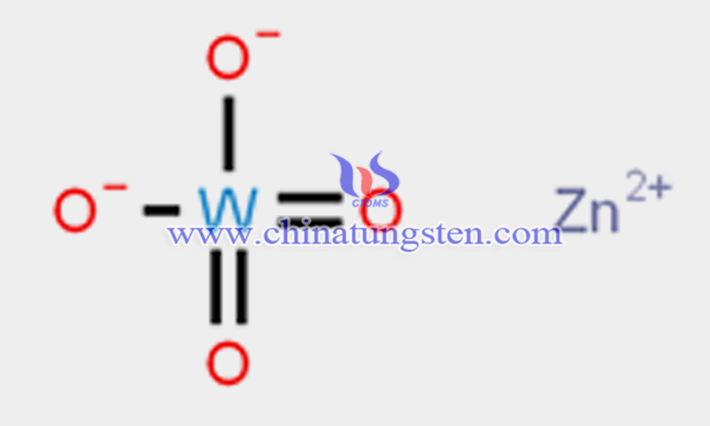
From a chemical composition perspective, zinc tungstate, also known as zinc tungsten oxide, is a tungstate made of tungsten, zinc, and oxygen. It is a covalent compound formed by divalent zinc ions (Zn2?) and tungstate ions [(WO?)2?], exhibiting a wolframite structure. Its English name is zinc tungstate, with the chemical formula ZnWO?, molecular weight 313.22, and CAS number 13597-56-3.
From a physicochemical properties standpoint, ZnWO? is a white powder with mild irritant properties, 99.9% purity, a density of 7.41 g/cm3, a melting point of 1,220°C, insolubility in water, and characteristics such as a wide bandgap, high excitation energy, strong UV response, high catalytic activity, short radiation length, and robust radiation damage resistance.
Regarding production processes, the preparation of ZnWO? involves the following steps: First, mix zinc nitrate with polyvinylpyrrolidone (PVP) or a surfactant blend of PVP and SDBS (sodium dodecylbenzene sulfonate) in water, stirring until uniform. Then, add sodium tungstate to the solution and stir thoroughly. Next, transfer the mixture to an autoclave, adding water until it reaches 80% of the reactor’s total volume. Finally, conduct a heating reaction, allow natural cooling after the reaction, and wash the product with deionized water and anhydrous ethanol, followed by drying.
In terms of applications, zinc tungstate can serve as a scintillator material in X-ray computed tomography scanners and security inspection equipment. It is also used as a semiconductor-type catalyst for the degradation of halogenated benzene derivatives and water pollutants.




