From a chemical composition perspective, unlike sodium tungstate (Na?WO?) and potassium tungstate (K?WO?), where the cations (Na? and K?) are alkali metals, the cation in calcium tungstate (Ca2?) is an alkaline earth metal. This leads to slight differences in structure, physicochemical properties, production processes, and applications. Note: Sodium and potassium are both alkali metal elements.
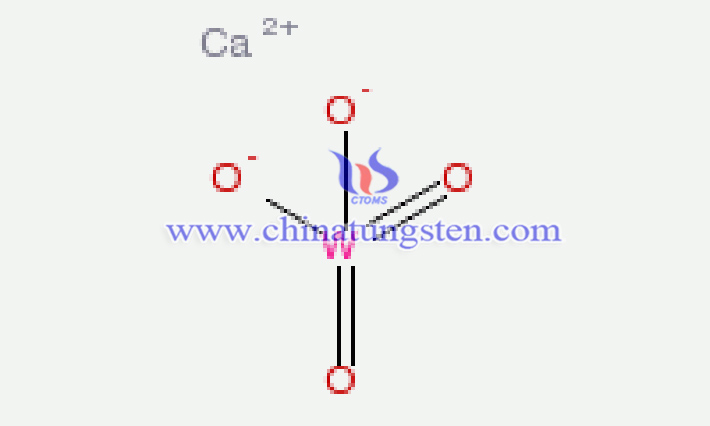
From a definitional standpoint, calcium tungstate, also known as "synthetic scheelite," is a tungstate composed of divalent calcium ions (Ca2?) and tungstate ions [(WO?)2?]. It is a typical self-activated luminescent material with a tetragonal crystal structure, known in English as calcium tungstate, with the chemical formula CaWO?, molecular weight 287.93, and CAS number 7790-75-2. Note: In CaWO?, the oxidation state of W is +6; the structures of Na?WO? and K?WO? are both monoclinic.
From a physicochemical properties perspective, CaWO? appears as a white powder with mild irritant properties, a density of 6.06 g/mL, a melting point of 1,580°C, a refractive index of 1.93, slight solubility in water and ammonium chloride solution, and decomposition in hot hydrochloric acid. It exhibits good catalytic activity and luminescent properties.



Regarding production methods, CaWO? can be prepared using solid-state reaction, hydrothermal, ultrasonic irradiation, microwave radiation, precipitation, or gel methods. However, these methods have limitations, such as harsh reaction conditions, low production efficiency, and uniform product morphology. An alternative preparation process for calcium tungstate involves: 1) Adding sodium tungstate to a β-cyclodextrin solution to obtain solution A; 2) Mixing a calcium nitrate solution with solution A at a 1:1 volume ratio, followed by stirring the reaction; 3) Precipitating, washing, and drying the resulting product to yield twinned spherical calcium tungstate microcrystals.
In terms of applications, calcium tungstate can be used to produce other tungsten derivatives such as ammonium paratungstate, tungsten oxide, tungsten alloys, and cemented carbides. It serves as a blue phosphor in oscilloscopes and a scintillator for detecting X-rays and γ-rays in medical applications. Additionally, it can be used as a laser matrix material.



