Like calcium tungstate (CaWO?), magnesium tungstate is an inorganic compound composed of a transition metal, an alkaline earth metal, and a non-metal element, exhibiting excellent optical, electrical, and magnetic properties, making it widely applicable in optoelectronics and chemical industries. Note: Both calcium and magnesium are alkaline earth metals, with atomic numbers 12 and 20, respectively.
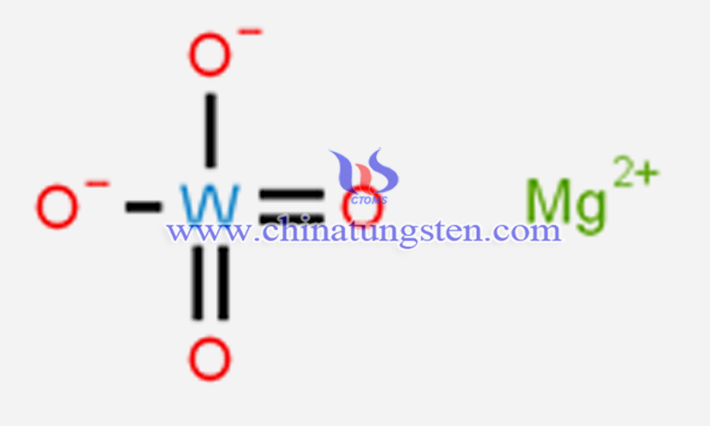
From a chemical composition perspective, magnesium tungstate is a self-activated fluorescent material made of tungsten, magnesium, and oxygen. It is a tungstate formed by divalent magnesium ions (Mg2?) and tungstate ions [(WO?)2?], with a monoclinic or tetragonal crystal structure. Its English name is magnesium tungstate, with the chemical formula MgWO?, molecular weight 272.14, and CAS number 13573-11-0.
From a physicochemical properties standpoint, MgWO? appears as a white powder with mild irritant and toxic properties, a density of 6.89 g/mL, insolubility in water and alcohol, solubility in acids, and good catalytic and luminescent properties.
In terms of applications, magnesium tungstate can be used to prepare red phosphors, catalysts, functional ceramic materials, and coating filler modifiers. However, the preparation methods vary depending on the product.

The preparation steps for magnesium tungstate red phosphor are as follows: Add a magnesium salt solution and a rare earth ion compound solution to a tungstate solution, adjust the pH, and allow the reaction to form a white turbid liquid. After vacuum filtration, dry, grind, and calcine the filtered sample to obtain the product.
The preparation steps for a magnesium tungstate catalyst are as follows: Mix nanosized magnesium tungstate semiconductor material with the organic dye methyl orange II in a container, stirring under light-proof conditions. Place the container with the mixture into an ultrasonic device for ultrasonic treatment to catalyze the degradation of methyl orange II.



